Investigational Therapies
for Patients
ImmunityBio has developed investigational immunotherapy products that are designed to help strengthen each patient’s natural immune system, potentially enabling it to outsmart the disease and eliminate rogue or infected cells.
Harness Nature’s First Responders™
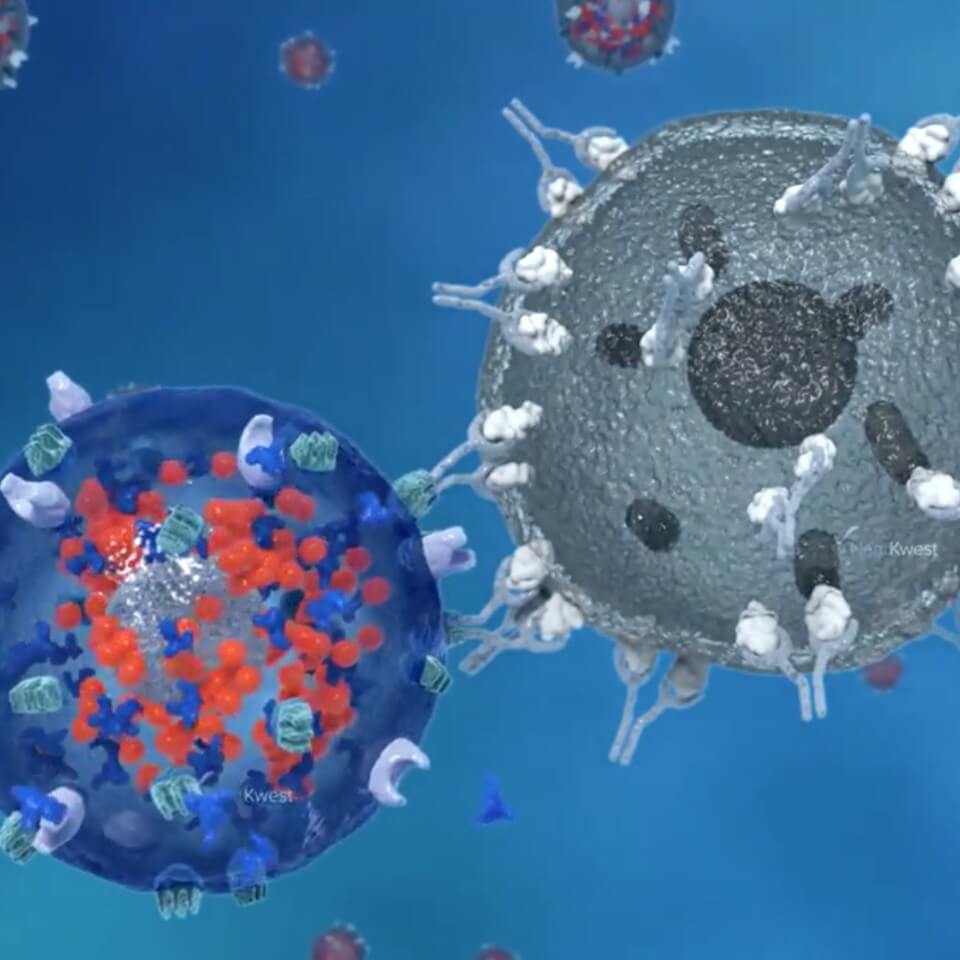
The immune system is a biological wonder that has evolved to effectively fight many infections and diseases. Thanks to advanced research, scientists are creating new therapies that harness this natural system and enhance it to fight cancers and other diseases that can outwit the immune system. At ImmunityBio, we have developed an orchestrated “triangle offense” approach that takes advantage of the natural immune system’s power to attack and kill cancerous or virus-infected cells.
The core of our offense is our activated Natural Killer (aNK) cells. Natural NK cells, part of the innate immune system, are always on alert and ready to defend our bodies from many kinds of infection or rogue cells, such as those that cause cancer. The second aspect of our triangle offense relies on the adaptive immune system, which includes specific white blood cells called ‘T cells’. T cells engage in a deeper, prolonged attack on infected or rogue cells. T cells are a kind of reserve player that have to be activated by the innate immune system. The third piece of our strategy employs chimeric antigen receptors (CARs), which are precision attackers that seek out specific and unique markers (antigens) that exist on the surface of cancer cells.
Our unique investigational PD-L1 t-haNK™ platform combines all three of these attack modes in a single cell that can be administered by a physician (investigator) in an outpatient clinic. These bioengineered NK cells are designed to directly attack rogue cells, stimulate and recruit the adaptive immune system’s cells so they learn what to attack, and home in selectively on cancer cells by use of CARs. In PD-L1 t-haNK cells, the CAR targets the ‘checkpoint’ protein programmed death domain ligand-1 (PD-L1) that can disable the immune response if not blocked. This triangle offense approach is one of the most promising therapeutic approaches for cancer treatment that has emerged in recent years, especially for patients with difficult-to-treat cancers. Checkpoint-directed and CAR-T therapies when used alone target only the adaptive, and not the innate, immune system and despite the progress in immunotherapy represented by the development of checkpoint blockers, they fail to successfully treat approximately 70% of solid tumors. Our PD-L1 t-haNK cells are distinguished from other immune-based therapies by their ability to coordinate the innate and adaptive responses that build the ‘immunological memory’ needed for robust and durable responses.
The ImmunityBio Hypothesis
The activation of the innate immune system is key to the development of immunological memory.
Investigational Therapies for Difficult Diseases

We are Committed to Increasing Diversity in Clinical Trials
Latest News & Events
ImmunityBio Announces Positive Overall Survival Results of Anktiva Combined With Checkpoint Inhibitors in Non-Small Cell Lung Cancer; Meeting Scheduled with FDA to Discuss Registration Path for ANKTIVA in Lung Cancer
QUILT 3.055 trial completed and shows median overall survival almost double that of standard of care chemotherapy in...
ImmunityBio Announces FDA Approval of ANKTIVA®, First-in-Class IL-15 Receptor Agonist for BCG-Unresponsive Non-Muscle Invasive Bladder Cancer
Designated an FDA Breakthrough Therapy, the novel immunotherapy ANKTIVA activates the body’s natural killer (NK) and...
NIAID-Sponsored Study Shows N-803 Combined with Neutralizing Antibodies Could Lead to Sustained HIV Viral Control After Discontinuation of Antiretroviral Therapy
CULVER CITY, Calif., March 6, 2024 — ImmunityBio (NASDAQ: IBRX), a clinical-stage immunotherapy company, today...